Disclaimer: This article includes affiliate links. If you choose to purchase any of the products we have discussed in this article, we may receive a small, and much appreciated commission.
Flashlight performance is all about the battery powering it, and the best flashlight battery type is the one that matches your needs as well as your battery maintenance knowledge and ability. Knowing the characteristics of your battery cells will help you better understand the characteristics of your flashlight. Here is a flashlight battery comparison to help you noodle through the jargon.
Primary vs Secondary (Rechargeable) cells
Choosing between Disposable and Rechargeable batteries to power your flashlight comes down to cost, convenience, and performance.

Disposable (Primary Cell)
Disposable batteries are also referred to as Primary Cells and are typically the Alkaline batteries that we usually use around the house or see at the department store. These are non-rechargeable and must be replaced once they are discharged. Primary Cell batteries are convenient and allow for you to rapidly power your device with fresh cells once the current ones are depleted. However, the costs of new cells starts to add up over time, and batteries are not ecologically friendly so must be disposed of properly.
Rechargeable (Secondary Cell)
Rechargeable cells are sometimes referred to as Secondary Cells. The constant and growing need to power portable technology such as laptops, cell phones and now cars has really evolved rechargeable battery technology. Performance and capacity, lower cost of ownership (dollar savings!) along with convenience of recharging have made Rechargeable batteries the best choice for many applications.
Rechargeable cells can be recharged (cycled) hundreds or even thousands of times. Yes, a single rechargeable cell can theoretically replace 1000 disposable batteries! The positive impact on the environment and your wallet can be huge when you choose and use rechargeable cells.
Voltage, Amps (Current) and Resistance
You probably see electronics described in terms of Voltage most often, but Voltage is only one of the three legs of the electricity stool. Understanding the relationships between Voltage, Amps and Resistance will help you understand some of the battery specifications.
A water example is often used to describe the relationship between Voltage, Amps and Resistance.
- Voltage is the water pressure in a pipe
- Current is the volume of water travelling through the pipe
- Resistance is the size of the pipe
The thing to remember is as you increase Voltage, you also increase Current which allows for higher performance for components designed for it.
Battery voltages depend on the chemistry of the battery, where size is the indicator of capacity (a ‘water tank’ to continue our plumbing analogy). For example, a single ‘AA’ alkaline battery has the same Voltage as a ‘D’ cell alkaline battery (1.5 volts), although the ‘D’ cell has much more capacity.
Voltages are additive, and to get higher voltages manufacturers will connect multiple ‘cells’ to multiply the output. If you peel open a classic square ‘nine-volt’ battery, you will often find a six-pack of small alkaline cells inside (a not-so-common ‘AAAA’ size) at 1.5 volts apiece, giving it a 9-volt output.
Voltage Curve
Battery voltage does not remain static as it is used. The amount of voltage remaining in a cell changes as the cell is discharged, and the curve of that line (voltage curve) can be shown on a graph. The amount of change in voltage over time is dependent on the chemistry of the battery. Voltage in Alkaline cells drops almost directly with usage (i.e. when the battery is halfway depleted, the voltage is about half of normal), while other cells maintain high output right up until the cell is depleted.
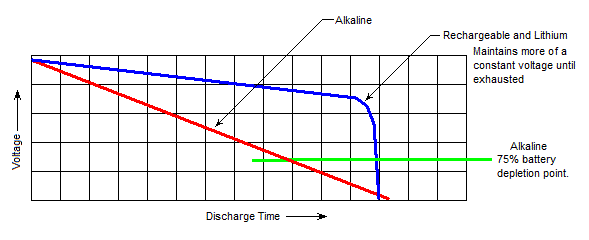
This is an important consideration for electronics that have a minimum operating voltage as there will be cases where a cell can no longer power a flashlight or other piece of equipment even though it still has plenty of ‘juice’ left. When I worked as a wedding photographer I went through a ton of disposable ‘AA’ cells in my flash, which then had a long second life powering kids toys and cheap flashlights.
NiMH or Li-Ion rechargeable cells, or Lithium primary cells, can last a bit longer in some electronics than Alkaline cells even if they have a smaller capacity. This is because they produce more consistent voltage over their life than Alkaline batteries.
Shelf Life
All batteries suffer from self-discharge. Over time they lose their charge from a few percentage points a year to 15% or more in a month. Once again this is dependent on the chemistry of the battery. Primary cells have a longer shelf life than rechargeable cells and can be stored for years with minimal loss of voltage. If you are constantly using (and re-charging) your cells such as in a working flashlight then a high self-discharge is not a factor to you. However, if you are storing your flashlight in an emergency kit that will hopefully be on a shelf for months or years then using primary cells is probably the way you want to go.
Battery Chemistries
From the original dry cells to today’s Li-Ion options, battery chemistries have come a long way. In today’s market you can choose the best battery chemistry for your needs. Here are the options you are most likely to find and what the advantages are for each.
Alkaline
Specs:
- Nominal Cell Voltage: 1.5v per cell
- Common Sizes: ‘AAA’, ’AA’, ‘C’, ‘D’, ‘nine-volt’ (six 1.5 volt cells making it 9 volts)
- Typical chemistry: Zinc and Manganese Dioxide
- Operating Temperature: -20C to +55C
- Self Discharge Rate: 2-3%/year
- Non-rechargeable
Alkaline cells are common and easy to find. When I was traveling the world in my younger days I made a point to have a camera that ran on standard ‘AA’ batteries because they could easily be found anywhere I traveled.
In all batteries the actual voltage may vary slightly so ‘nominal voltage’ is used to indicate the general voltage of the cell. Fresh alkaline cells have a nominal cell voltage of 1.5 volts. As they discharge through use they have a sloping voltage curve, dropping slowly to about 1 volt then very quickly after that. The lower voltage of an older battery has an obvious dimming effect that is easy to see in an incandescent flashlight.
Alkaline batteries that are depleted, or that overheat, are prone to leaks. When you store equipment with batteries inside there is often a small trickle ‘parasitic charge’ that drains the cell faster than normal self-drain. This causes leaks and corrosion that are often found when you pull out flashlights that have been in a drawer for a few months. It is always best to remove alkaline cells from equipment that you are going to store any length of time.
Being a disposable Primary Cell, consistent use of alkaline cells can be expensive over time and leave a large footprint on the environment.
The low self-discharge rate of Alkaline cells will allow them to be stored in a cool, dry place for a long time. They can maintain 80% of their charge after 5 years. Alkaline batteries are best for low-drain devices such as clocks or remote controls.
Having problems with your batteries? Check out our article explaining Why Batteries Leak

NiMH (Nickel-Metal Hydride)
Specs:
- Nominal Voltage: 1.25v per cell
- Common Sizes: ‘AAA’, ’AA’, ‘C’, ‘D’, ‘nine-volt’
- Battery Chemistry: Nickel Metal Hydride Battery
- Operating Temperature: -20C to +60C
- Self-Discharge Rate: 5-20% on day 1, 0.5%-5% per day after that (significant increase in hot environments)
- Rechargeable
Nickel-Metal Hydride (NiMH) batteries can be found in the same sizes as standard Alkaline batteries. They provide a lower initial nominal voltage than Alkaline’s (1.25v vs 1.5v), but maintain their working voltage for longer than Alkalines due to their flatter discharge curve. NiMH cells also have a higher capacity than Alkalines, often by 2 or 3 times.
These rechargeable cells do typically have a very high self-discharge rate and usually need charging immediately before use if they have been sitting for a few weeks. NiMH cells don’t suffer from ‘charge memory’ like other rechargeables such as NiCd (Nickel-Cadmium). However they can suffer from ‘voltage depression’ which has a very similar effect in a reducing the capacity of the cell over time.
NiMH cells are great for high-drain electronic devices such as cameras and camera flashes due to their relatively flat discharge curve. Because of their high self-discharge rate they do not do well in low-drain devices such as clocks or smoke alarms. They also leave a much smaller environmental footprint with the ability to be recharged hundreds of times.

Lithium
Specs:
- Nominal Voltage: 3v per cell
- Common Sizes: CR123A, CR2, ‘AA’
- Battery Chemistry: Lithium-metal
- Operating Temperature: -40C to +60C
- Self-Discharge Rate: 2-3% per year
- Non-Rechargeable
Lithium primary cells are usually found in their own standard 3 volt sizes such as CR123A or CR2. There are some types of Lithium cells in standard sizes of ‘AA’ or ‘nine-volt’, but they have a different chemistry or embedded circuitry that brings their voltage down to 1.5 volts instead of the normal 3 volts. Such batteries are suitable replacements for Alkaline cells as they are electrically and physically compatible. There may be times when the advantages of Lithium such as cold weather durability and flatter discharge curve than Alkalines will be an advantage.
Lithium cells will last for years when stored in a cool, dry place. They have a very low self-discharge rate of 2-3% per year and can last 10 years in storage.
Standard Lithium cells have an operating temperature starting at -40C, making them better suited for cold-weather climates than other batteries which normally start at -20C.
Higher energy density and low self-discharge rate make them perfect for applications where your need high performance after a prolonged storage or standby mode such as in smoke alarms, emergency flashlights or outdoor game cameras.
Being non-rechargeable and disposable they have a similar environmental impact as Alkaline cells. Lithium cells are also more expensive than Alkaline so the costs can add up even quicker with regular use.

Li-Ion
- Nominal Voltage: 3.7v per cell
- Common Sizes: 18650 (2x CR123A Size), RCR123 (1x CR123A), 14500 (AA size)
- Battery Chemistry: Lithium-Ion
- Operating Temperature: -20C to +60C
- Self-Discharge Rate: 2-3% per year
- Rechargeable
Li-Ion cells are some of the most common cells found today in portable electronics. They come in their own size standards where the name of the cell describes the dimensions. For example: a ‘18650’ translates to 18mm in diameter, 65mm in length, and the ‘0’ indicates it is round.
These cells have the highest energy density of this list at a nominal voltage of 3.7 volts per cell. They operate in normal temperature ranges and have a relatively low self-discharge rate.
Be cautious here. There are several Li-Ion batteries which are the same dimensions as the standard sizes that you are used too. However, they are typically not interchangeable because of Li-Ion batteries have much higher voltage than Alkaline. The difference may damage your equipment.
Here is a list of standard vs Li-Ion size equivalents:
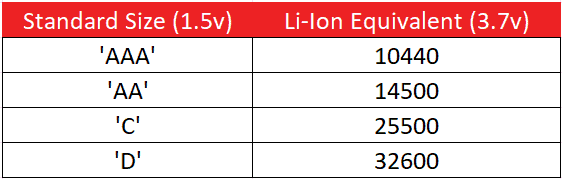
The most common size you will see for a rechargeable Li-Ion cell is the 18650. These cells can usually be replaced with two Lithium CR123A primary cells in a pinch.
There are a number of different Li-Ion battery chemistries that fall into two categories: Protected and Unprotected.
Protected
Most commercially available Li-Ion cells have a Lithium Cobalt chemistry and are referred to as ‘ICR’. They have a protection circuit is attached to the front of the cell and regulates discharge, protects against over-discharge and protects against overcharging. The protection circuit adds a bit of length to the cell, causing these cells to be referred to as ‘button tops’.

Unprotected
Another Li-Ion chemistry is Lithium Manganese, or IMR. The IMR chemistry is not as volatile as others and is sold without the protection circuit common to ICR cells, making them ‘unprotected’ – also referred to as ‘flat top’. These unprotected cells have higher output – sometimes far beyond the nominal 3.7 volts of button tops – which translates to higher performance. Some tactical flashlights recommend or even require unprotected cells such as the NITECORE TM03 or the Nitecore Concept 1 for maximum performance. When using these batteries look for a flashlight with an integrated voltmeter or protection circuit, as completely draining these cells will permanently damage them.
Li-Ion cells are more volatile than Alkaline or NiMH. They require a bit more care and feeding than other cells and can easily be damaged or even explode if not charged, discharged or stored properly. Some flashlights have protection circuits and a charger built right into the unit to manage these details for you. If yours doesn’t then always utilize at high quality smart charger made for Li-Ion cells and be cautious about discharging them too much.
Li-Ion cells need to be stored in an partially charged state to prolong their life. If they are discharged and stored under three volts per cell they can be ruined and should be discarded.
The higher voltage of rechargeable Li-Ion cells make them a great choice for high use, high drain electronics. This includes flashlights, phones and cameras that are designed for them. The higher performance does come at a cost. Li-Ion cells, especially unprotected ones, do require some more attention and knowledge than typical Alkaline or even NiMH batteries for safe handling.
Best Batteries by Cell Type
Check out these articles for our Flashlight recommendations by Battery Type:
Conclusion
As you can see the best battery chemistry for your flashlight will be determined by your use. How much performance do you need? What size of light do you need? Does it need to be rechargeable? Do you need to be able to replace the cells when they are dead? Are you using this in very cold or hot weather? All are factors in determining the best battery for your flashlight or even other uses.
